Nature is heavy: only one needle is needed to make the immune system "rejuvenate", and the new anti-aging therapy restores young immune characteristics.
Original biological world
Written by Cong Wang
Edit Wang Duoyu
Typesetting and writing in water
A single hematopoietic stem cell (HSC) can produce all blood cells and renew itself to maintain the stem cell bank in life. Hematopoietic stem cells have functional heterogeneity and may have different contributions to the lineage of lymphoid and myeloid cells. There are at least two subtypes of hematopoietic stem cells (HSC)-BAL-HSC (lymphomyeloid balanced hematopoietic stem cells) and my-HSC (myeloid biased hematopoietic stem cells). The former can produce lymphocytes and myeloid cells in a balanced way, while the latter tends to mainly produce myeloid cells. Compared with bal-HSC, the proportion of my-HSC will increase with age.
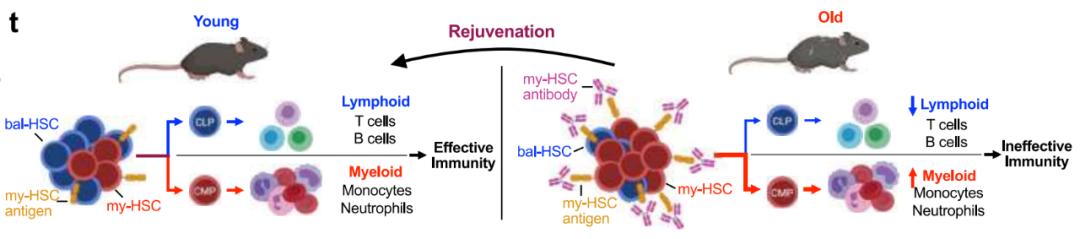
This age-related transition from bal-HSC to my-HSC reduces lymphocyte production and increases myeloid cell production, which leads to many pathological phenomena in the elderly, including adaptive immune decline, inflammation and various myeloid-related diseases. This also suggests that if we can develop a treatment method to make the immune system return to a younger state of more hematopoietic stem cells (HSC) and less my-HSC, so as to produce lymphocytes and myeloid cells in a more balanced way, it may solve the age-related pathological phenomenon.
On March 28th, 2024, Irving Weissman team of Stanford University and Kim Hasenkrug team of National Health Research published a research paper entitled: Deleted Myeloid-biased Haematopoeic Stem Cells rejuvenating aged immunity in the journal Nature.
The research team has developed an antibody therapy, which can increase the proportion and quantity of bal-HSC by targeted removal of my-HSC in old mice, make the old immune system return to a young state, restore young immune characteristics, reduce the decline of age-related immune function, and improve the protective immunity of old mice against virus infection.

The aging of immune system is characterized by the decline of lymphopoiesis and adaptive immunity, the increase of inflammation and myelopathy. Age-related changes in self-renewing hematopoietic stem cell (HSC) subsets are considered to be the basis of these phenomena. In youth, HSC(bal-HSC), which has the balance between lymphocytes and myeloid cells, is dominant, thus promoting the lymphopoiesis needed to start adaptive immune response and limiting the production of myeloid cells that may promote inflammation. However, in the old age, the proportion of hematopoietic stem cells (my-HSC) produced by biased myeloid cells increased, which led to the decrease of lymphatic hematopoiesis and the increase of myeloid hematopoiesis.
Two subgroups of mouse hematopoietic stem cells (HSC), bal-HSC and my-HSC, can be distinguished according to the expression level of CD150. bal-HSC has low expression of CD150, while my-HSC has high expression of CD150. Besides CD150, there are more than a dozen cell surface proteins, such as CD41, CD61, CD62p and NEO1, which are highly expressed on the surface of my-HSC. In order to determine the best target on my-HSC, the research team used antibodies and flow cytometry to evaluate their levels on the surface of my-HSC and bal-HSC cells.
The results showed that compared with bal-HSC, the most abundant cell surface proteins on my-HSC were CD41, CD62p and NEO1. The research team used them together with CD150 as candidate target antigens of antibody-mediated my-HSC, and independently developed antibodies against these target antigens to target depletion of my-HSC.
The results showed that antibody targeting CD150 was enough to deplete my-HSC in vivo. In order to further improve the clearance efficiency of my-HSC mediated by antibody, the research team added anti-CD47 antibody and/or anti-KIT antibody on the basis of anti-CD150 antibody, which can produce stronger clearance effect of my-HSC after about one week, and increase the proportion and absolute number of bal-HSC in bone marrow. These results show that the combination of anti-CD150 antibody, anti-CD47 antibody and anti-KIT antibody is the most effective clearance scheme for my-HSC.
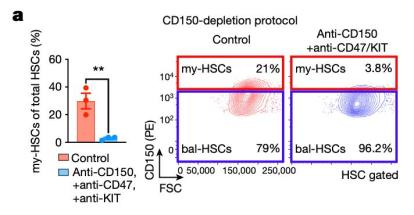
This study further shows that targeting and eliminating my-HSC in vivo can restore the youthful characteristics of the immune system, such as more common lymphocyte progenitor cells and other immune cells, and at the same time reduce the age-related inflammatory markers with decreased immune function. In addition, the treated aged mice can produce better immune response to virus infection.
The changes of hematopoietic stem cells (HSC) between mice and humans during aging and the conservativeness of the amplification of my-HSC indicate that this preclinical study can support the development of clinical treatment programs to help the human immune system recover its youthful state, thus having a wide impact on many age-related problems. Of course, further preclinical and clinical studies are needed to determine the feasibility of this immunotherapy in humans.
It should be pointed out that increasing the production of lymphocytes in old age can make the immune system recover its youthful characteristics and enhance its anti-infection ability, but it may also increase the risk of tumor growth (such as leukemia). Therefore, it is necessary to strengthen immune monitoring to reveal the long-term impact of immunotherapy to restore its youthful characteristics.
Paper link:
https://www.nature.com/articles/s41586-024-07238-x
Read the original text